Yu, T., Koppetsch, B.S., Pagliarani, S., Johnston, S., Silverstein, N.J., Luban, J., Chappell, K., Weng, Z., and Theurkauf, W.E. (2019). The piRNA Response to Retroviral Invasion of the Koala Genome. Cell. is online today!!!
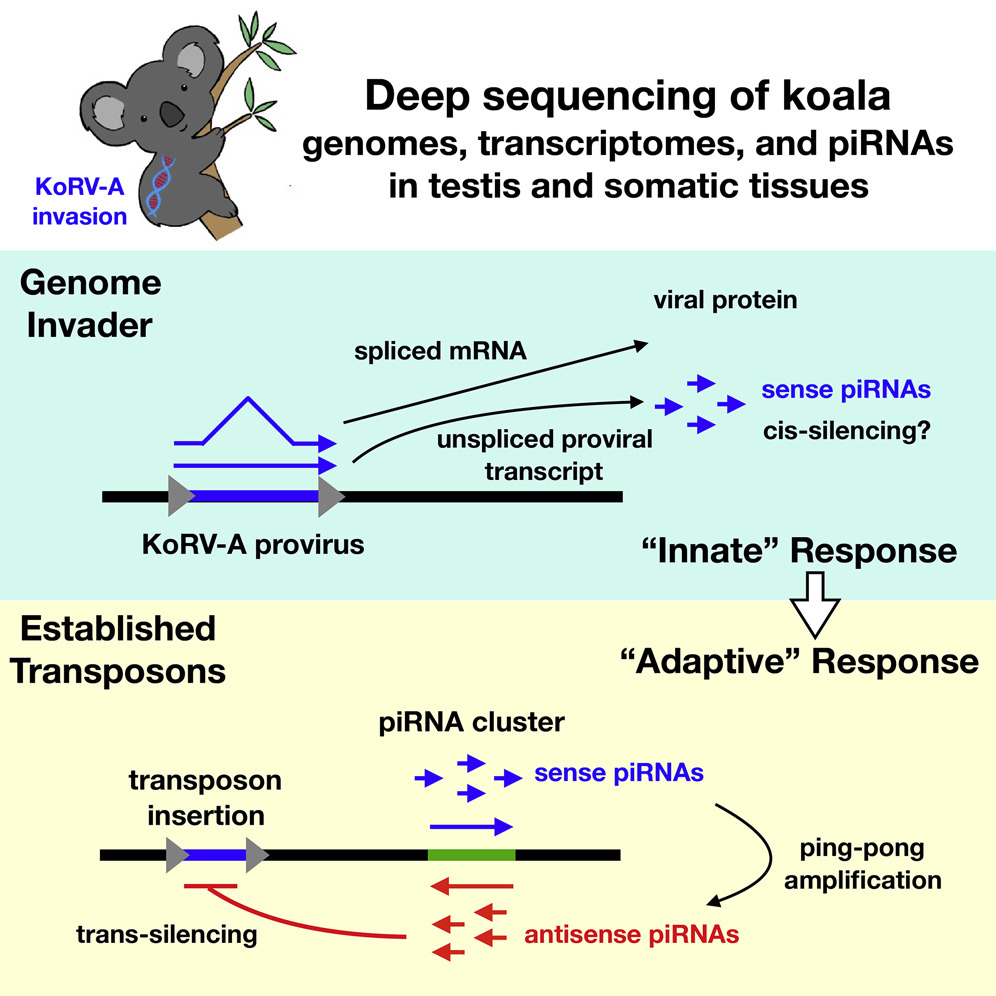
Fruit fly researcher Bill Theurkauf, our neighbor here in the Program in Molecular Medicine at UMass Medical School, along with UMass Med bioinformatics guru Zhiping Weng, has been studying the mechanisms by which piRNAs are generated in the germ cells of Drosophila. It is believed that the piRNAs are a sequence-specific defense system that protects the germ cell genome from DNA-damage caused by jumping genes like endogenous retroviruses. Similar piRNAs are found in mice and humans, and it is important to remember that 8% of the human genome is composed of retrovirus sequence.
Bill heard that there was an epidemic in Koalas caused by KoRV-A, a retrovirus that causes an immunosuppressive illness with parallels to AIDS. What caught Bill’s interest was the fact that, unlike HIV-1, KoRV-A was not only transmitted horizontally, but also vertically through the germline.
As described in a manuscript that was published today, Bill then teamed up with colleagues in Brisbane to determine whether the germ cells of infected Koalas generated piRNAs specific for KoRV-A. Indeed they do, though, unlike “mature” piRNAs, the piRNAs targeting KoRV-A were only + sense-stranded. This suggests that the piRNA system was acting as an antiviral system that acts directly on the KoRV-A transcripts to degrade them.
Another interesting detail is that only unspliced KoRV-A transcripts were detected among the piRNAs, despite 5-fold higher levels of the spliced transcripts. This suggests that the unspliced transcript, a molecular feature typical of all retro-elements is a PAMP, or danger signal, that is recognized by our cells antiviral machinery. Similar ideas about unspliced transcripts from retroelements have been reported by the lab of Hiten Madhani regarding siRNAs in Cryptococcus, and, separately, by the Gummuluru Lab and by the Luban Lab concerning unspliced transcripts of HIV-1 in human immune cell types.
Noah Silverstein, graduate student in the Luban Lab, contributed to the piRNA manuscript by identifying and collecting mouse strains bearing endogenous retroviruses in their genome that are close cousins to KoRV-A. Like the koalas, these mice also generated +-stranded piRNAs in their germ cells.
The Theurkauf, Weng, and Luban Labs here at UMass Medical School, along with our Brisbane colleagues Johnston and Chappell, will continue working to address the many questions that remain to be answered about these piRNAs targeting retroviruses that are newly integrated into the mammalian genome.
Our paper is covered in an article in the New York Times, on the website for UMass Medical School, and on local TV in Brisbane.